null
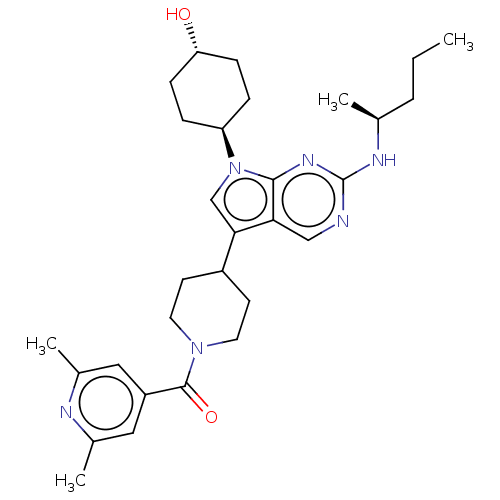
SMILES CCC[C@H](C)Nc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)C1CCN(CC1)C(=O)c1cc(C)nc(C)c1
InChI Key InChIKey=MSWOWUREQODTRO-LQGLAIQGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 497267
Found 4 hits for monomerid = 497267
Affinity DataKi: 19nMAssay Description:ATP competitive inhibition of TYRO3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of TYRO3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of N-terminal GST-tagged TYRO3 (453 to 890 residues) (unknown origin) cytoplasmic domain expressed in Sf21 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Activity Against Tyro3 Tyrosine Kinase.More data for this Ligand-Target Pair