null
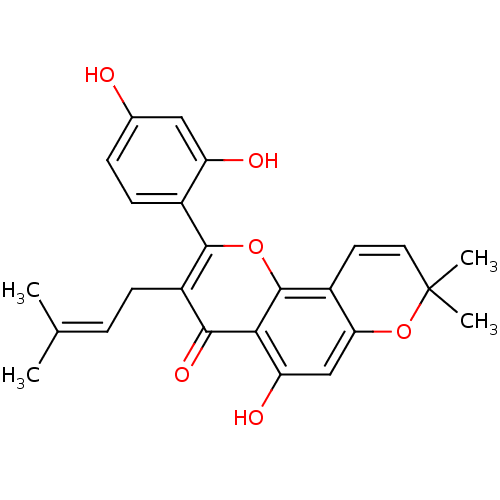
SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(oc2c3-[#6]=[#6]C([#6])([#6])[#8]-c3cc(-[#8])c2c1=O)-c1ccc(-[#8])cc1-[#8]
InChI Key InChIKey=XFFOMNJIDRDDLQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50242014
Found 2 hits for monomerid = 50242014
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Universidade Federal de Santa Catarina
Curated by ChEMBL
Universidade Federal de Santa Catarina
Curated by ChEMBL
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human PTP1B using p-nitrophenyl phosphate as substrate preincubated for 10 mins followed by substrate addition measured every minute fo...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Universidade Federal de Santa Catarina
Curated by ChEMBL
Universidade Federal de Santa Catarina
Curated by ChEMBL
Affinity DataIC50: 2.21E+4nMAssay Description:Inhibition of recombinant PTP1B catalytic domain (unknown origin) assessed as reduction in pNP formation using pNPP as substrate by absorbance based ...More data for this Ligand-Target Pair