null
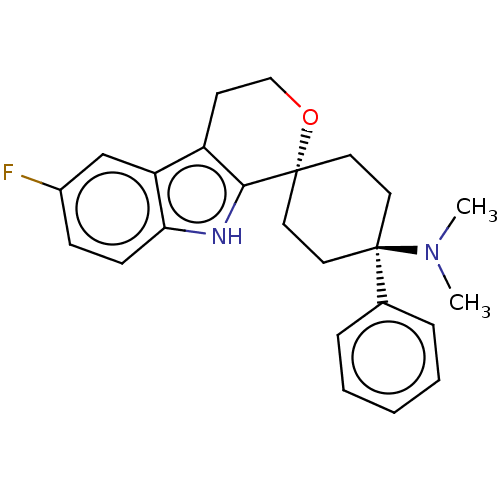
SMILES OC(=O)CC(O)(CC(O)=O)C(O)=O.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccc(F)cc12)c1ccccc1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50101095
Found 3 hits for monomerid = 50101095
TargetVoltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1S(Rat)
Pharmacokinetics
Curated by ChEMBL
Pharmacokinetics
Curated by ChEMBL
Affinity DataKi: 480nMAssay Description:Inhibition of rat brain cortex L-type Ca2+-channel phenylalkylamine siteMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1S(Rat)
Pharmacokinetics
Curated by ChEMBL
Pharmacokinetics
Curated by ChEMBL
Affinity DataKi: 800nMAssay Description:Inhibition of rat brain cortex L-type Ca2+-channel benzothiazepine siteMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1S(Rat)
Pharmacokinetics
Curated by ChEMBL
Pharmacokinetics
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of rat whole brain L-type Ca2+-channel dihydropyridine siteMore data for this Ligand-Target Pair