null
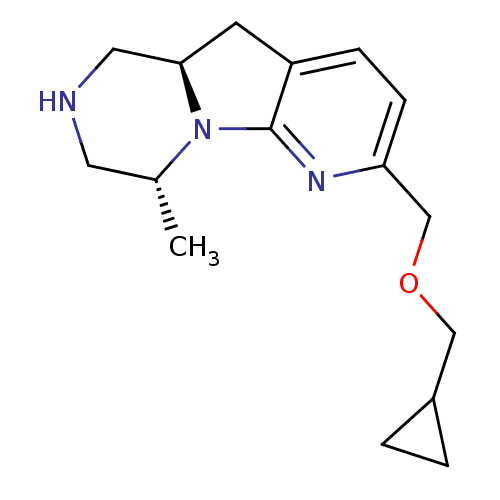
SMILES C[C@@H]1CNC[C@H]2Cc3ccc(COCC4CC4)nc3N12
InChI Key InChIKey=LJRLAJVWAFFRLV-IAQYHMDHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50179073
Found 2 hits for monomerid = 50179073
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
Affinity DataEC50: 15nMAssay Description:Efficacy against human recombinant 5HT2C receptor induced intracellular calcium mobilization in CHO cells by FLIPRMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
Affinity DataEC50: 23nMAssay Description:Efficacy against human recombinant 5HT2B receptor induced intracellular calcium mobilization in CHO cells by FLIPRMore data for this Ligand-Target Pair