null
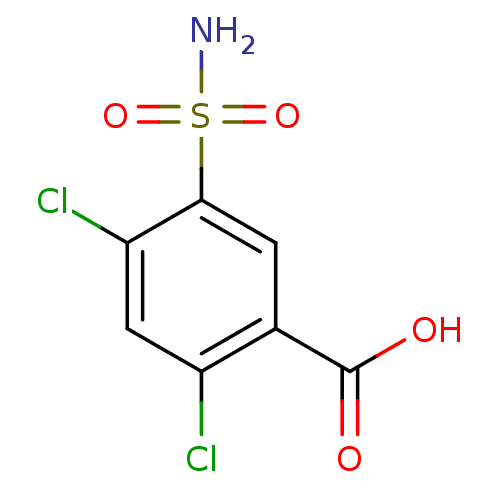
SMILES NS(=O)(=O)c1cc(C(O)=O)c(Cl)cc1Cl
InChI Key InChIKey=ZSHHRBYVHTVRFK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 13075
Found 14 hits for monomerid = 13075
Affinity DataIC50: 170nMpH: 7.4 T: 2°CAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 400nMAssay Description:The carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al....More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMpH: 7.4Assay Description:The carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of the 4-nitrophenylacetate (NPA) to the 4-nitrophenylate...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMpH: 7.4Assay Description:The carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of the 4-nitrophenylacetate (NPA) to the 4-nitrophenylate...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70E+3nMAssay Description:The method for determination of the Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:The method for determination of the Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. ...More data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of hydratase-activity of CA1 from human erythrocytes by CO2-hydration methodMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibition of hydratase-activity of human erythrocytes CA2 by CO2-hydration methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of esterase-activity of CA1 from human erythrocytes by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of esterase-activity of human erythrocytes CA2 by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70E+3nMAssay Description:Inhibition of CA1 from human erythrocytes by Lineweaver-Burke analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of human erythrocytes CA2 by Lineweaver-Burke analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:The carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al....More data for this Ligand-Target Pair