null
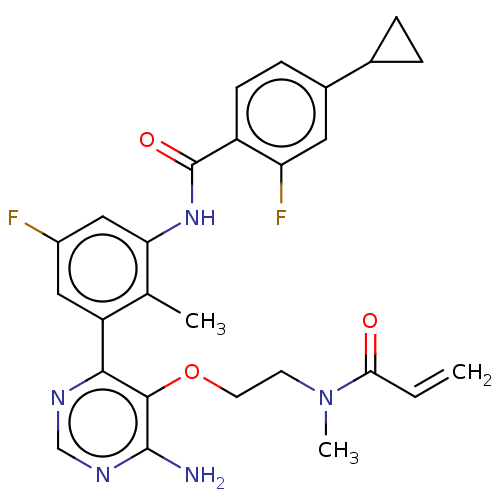
SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C
InChI Key InChIKey=CUABMPOJOBCXJI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 259407
Found 12 hits for monomerid = 259407
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of PIK4CB (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of BTK in human B cells assessed as reduction in anti-IgM/IL4-stimulated CD69 expression on B cells preincubated for 60 mins followed by a...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of BTK in human basophils assessed as reduction in anti-IgE mouse IgG1 antibody Le2-stimulated CD63 expression on basophil preincubated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of BSEP (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human ERG by Qpatch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair