null
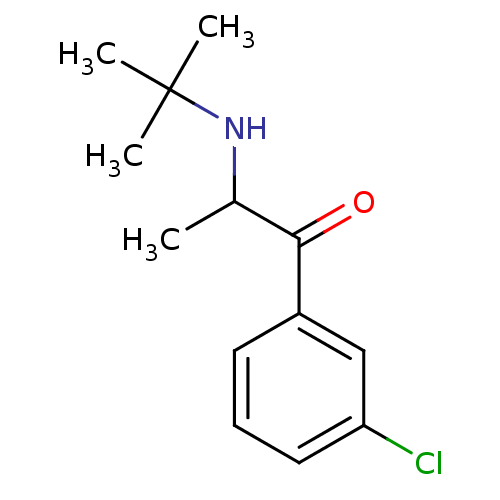
SMILES CC(NC(C)(C)C)C(=O)c1cccc(Cl)c1
InChI Key InChIKey=SNPPWIUOZRMYNY-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 47 hits for monomerid = 50048392
Found 47 hits for monomerid = 50048392
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 3.00E+5nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1-6(Homo sapiens (Human))
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 3.00E+5nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 2B7(Homo sapiens (Human))
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 3.00E+5nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 2B10(Homo sapiens (Human))
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 8.80E+4nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3/beta-4(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Antagonist activity at alpha3beta4 nicotinic receptor in human SH-SY5Y cells assessed as inhibition varbamylcholine-induced radiolabeled Rb+ influx a...More data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibition of [3H]norepinephrine reuptake at human NET expressed in HEK293 cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 660nMAssay Description:Inhibition of [3H]dopamine reuptake at human DAT expressed in HEK293 cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Antagonist activity at alpha4beta2 nicotinic receptor in human SH-EP1 cells assessed as inhibition varbamylcholine-induced radiolabeled Rb+ influx at...More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-4(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Antagonist activity at alpha4beta4 nicotinic receptor in human SH-EP1 cells assessed as inhibition varbamylcholine-induced radiolabeled Rb+ influx at...More data for this Ligand-Target Pair
TargetAcetylcholine receptor subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Antagonist activity at alpha-1-beta-1-gamma-delta nicotinic receptor in human TE671 cells assessed as inhibition varbamylcholine-induced radiolabeled...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Inhibition of DAT (unknown origin) assessed as transporter-mediated dopamine reuptakeMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Homo sapiens (Human))
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Non-competitive antagonist activity at alpha7 nAChR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.57E+3nMAssay Description:Inhibition of [3H]dopamine uptake at human dopamine transporter expressed in mouse N2A cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 658nMAssay Description:Inhibition of [3H]dopamine uptake at human DAT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibition of [3H]norepinephrine uptake at human NET expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Affinity DataIC50: 100nMAssay Description:Inhibition of [3H]serotonin uptake at human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataIC50: 1.23E+3nMAssay Description:Compound was tested for its ability to inhibit the neurotransmitter dopamine-DA reuptake system using [3H]dopamine as radioligandMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of [3H]paroxetine binding at serotonin transporter was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of dopamine (DA) reuptake using cloned human dopamine transporter was determinedMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 1.45E+3nMAssay Description:Inhibition of Norepinephrine (NA) reuptake using cloned human transporter was determinedMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Homo sapiens (Human))
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3/beta-2(Homo sapiens (Human))
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against Nicotinic acetylcholine receptor alpha3-beta2More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Antagonist activity against human alpha4beta2 nAChR in SHEP1 cells assessed as inhibition of carbamylcholine induced 86Rb+ effluxMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl...More data for this Ligand-Target Pair
Affinity DataIC50: 945nMAssay Description:Inhibition of [3H]dopamine reuptake at human DAT expressed in HEK293 cellsChecked by AuthorMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 443nMAssay Description:Inhibition of [3H]norepinephrine reuptake at human NET expressed in HEK293 cellsChecked by AuthorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human SERTexpressed in HEK293 cells assessed as inhibition of [3H]5HT reuptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibition of human NET expressed in HEK293 cells assessed as inhibition of [3H]NE reuptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 658nMAssay Description:Inhibition of human DAT expressed in HEK293 cells assessed as inhibition of [3H]DA reuptake after 10 mins by scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 3.24E+3nMAssay Description:Inhibition of [3H]-5-norepinephrine reuptake in human NET expressed in HEK293 cells by microbeta liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of [3H]-5-dopamine reuptake in human DAT expressed in HEK293 cells by microbeta liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 6.90E+4nMAssay Description:HERG: The pre- and post-compound hERG current was evoked by a single voltage pulse consisting of a 20 s period holding at −70 mV, a 160 ms step...More data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 3.24E+3nMAssay Description:Inhibition of re-uptake of [3H]-NE at human NET expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of re-uptake of [3H]-DA at human DAT expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 3.24E+3nMAssay Description:Inhibition of human NET expressed in HEK293 cells assessed as reduction in [3H]-NE uptake by micro beta scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human DAT expressed in HEK293 cells assessed as reduction in [3H]-DA uptake by micro beta scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of [3H]-5-dopamine reuptake in human DAT expressed in HEK293 cells by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 3.24E+3nMAssay Description:Inhibition of [3H]-5-norepinephrine reuptake in human NET expressed in HEK293 cells by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
TargetUDP-glucuronosyltransferase 1A4(Homo sapiens (Human))
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Affinity DataIC50: 1.52E+5nMAssay Description:Refer to Fisher et al., Drug Metab. Dispos., 28:560-566.More data for this Ligand-Target Pair