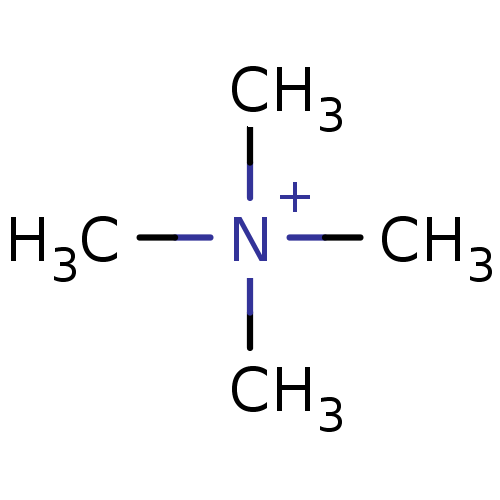null
SMILES C[N+](C)(C)C
InChI Key InChIKey=QEMXHQIAXOOASZ-UHFFFAOYSA-N
PDB links: 11 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50079455
Found 4 hits for monomerid = 50079455
Affinity DataIC50: 1.67E+6nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (TEA: 50 uM) in OCT2-expressing MDCK cellsMore data for this Ligand-Target Pair
TargetSolute carrier family 22 member 2(Homo sapiens (Human))
Anatomisches Institut der Bayerischen Julius-Maximilians-Universit£t
Curated by ChEMBL
Anatomisches Institut der Bayerischen Julius-Maximilians-Universit£t
Curated by ChEMBL
Affinity DataIC50: 1.50E+5nMAssay Description:TP_TRANSPORTER: inhibition of MPP+ uptake (MPP+: 1 uM) in Xenopus laevis oocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+5nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (TEA: 10 uM) in Xenopus laevis oocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+6nMAssay Description:TP_TRANSPORTER: inhibition of TEA uptake (TEA: 50 uM) in OCT1-expressing MDCK cellsMore data for this Ligand-Target Pair