null
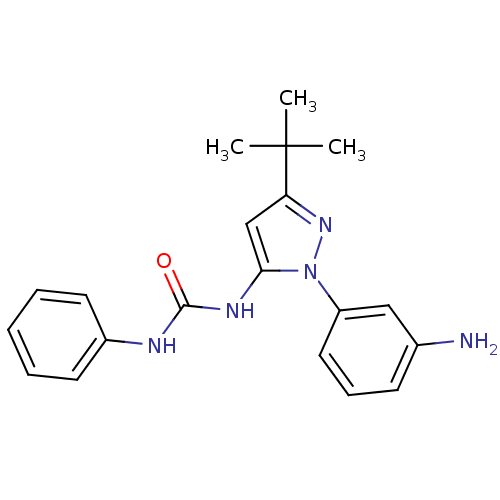
SMILES CC(C)(C)c1cc(NC(=O)Nc2ccccc2)n(n1)-c1cccc(N)c1
InChI Key InChIKey=DHNYNLNKNQJSHF-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50115216
Found 3 hits for monomerid = 50115216
TargetDiscoidin domain-containing receptor 2(Homo sapiens (Human))
Technical University of Dortmund
Curated by ChEMBL
Technical University of Dortmund
Curated by ChEMBL
Affinity DataIC50: 9.41E+3nMAssay Description:Inhibition of wild type DDR2 (unknown origin) preincubated for 30 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Chemical Genomics Centre of the Max Planck Society
Chemical Genomics Centre of the Max Planck Society
Affinity DataIC50: 440nMAssay Description:Displacemnt of N,N'-(2,2'-(3,3'-disulfanediylbis(2,5-dioxopyrrolidine-3,1-diyl))bis(ethane-2,1-diyl))bis(2-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureid...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Chemical Genomics Centre of the Max Planck Society
Chemical Genomics Centre of the Max Planck Society
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of p38alpha active form expressed in Escherichia coli BL21(DE3) cells by HTRF assayMore data for this Ligand-Target Pair