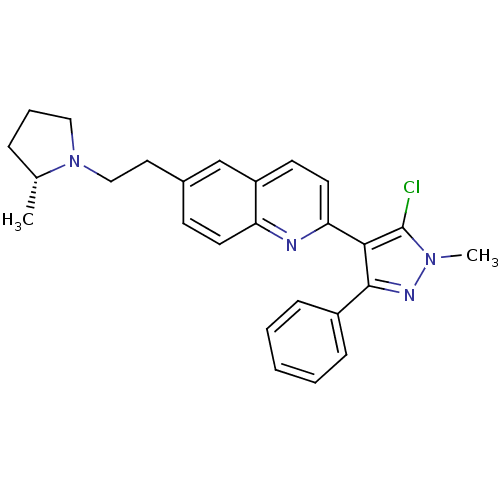
null
SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1c(Cl)n(C)nc1-c1ccccc1
InChI Key InChIKey=BHVLKNXTDWOMMA-GOSISDBHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50319548
Found 1 hit for monomerid = 50319548
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG by Competitive binding assayMore data for this Ligand-Target Pair