null
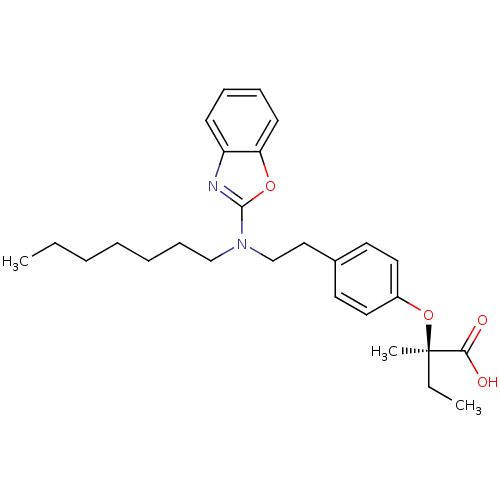
SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1
InChI Key InChIKey=QPKIEBNVIOELIR-HHHXNRCGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 28762
Found 2 hits for monomerid = 28762
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
Affinity DataKd: 270nMAssay Description:Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetryMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
Affinity DataKd: 685nM EC50: 73.3nMpH: 7.5 T: 2°CAssay Description:Kd values were obtained by incubating His-PPARgamma-LBD with biotinylated peptide, europium-labeled anti-histidine antibody, and allophycocyanin-labe...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)