null
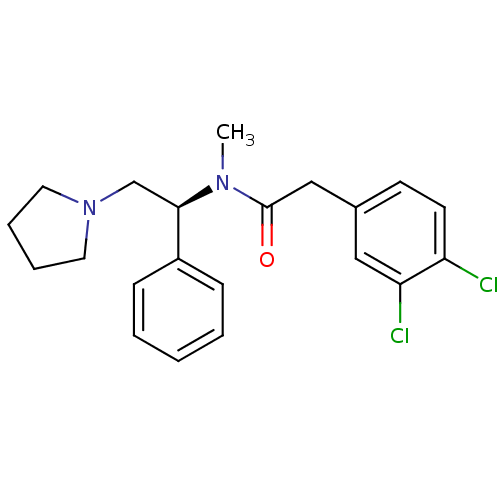
SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1
InChI Key InChIKey=AEJOEPSMZCEYJN-HXUWFJFHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50007344
Found 9 hits for monomerid = 50007344
Affinity DataKi: 0.0400nMAssay Description:Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.0430nMAssay Description:In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.0440nMAssay Description:Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0540nMAssay Description:In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibitory constant against human Opioid receptor delta 1 using [3H]diprenorphine as radio ligandMore data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Inhibitory constant against human Opioid receptor mu 1 using [3H]diprenorphine as radio ligandMore data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair