null
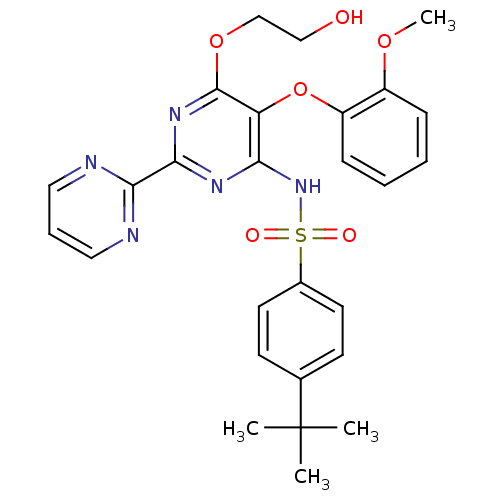
SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCO)-c1ncccn1
InChI Key InChIKey=GJPICJJJRGTNOD-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50061101
Found 8 hits for monomerid = 50061101
Affinity DataKi: 6.5nMAssay Description:Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Binding affinity towards Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibitory activity against human endothelin A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Inhibitory activity against human endothelin B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetEndothelin receptor type B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetEndothelin receptor type B(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 340nMAssay Description:Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:TP_TRANSPORTER: inhibition of Taurocholate uptake in membrane vesicle from Bsep-expressing Sf9-cellMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)