null
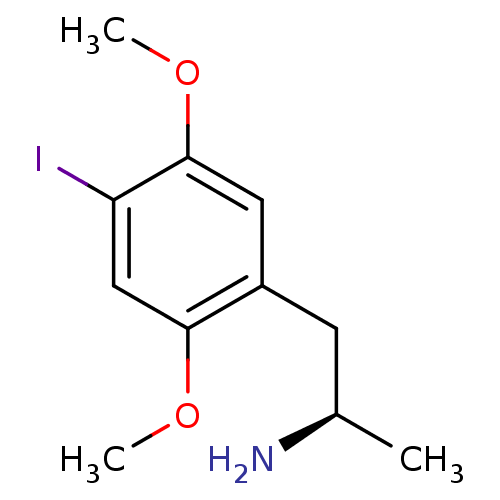
SMILES COc1cc(C[C@@H](C)N)c(OC)cc1I
InChI Key InChIKey=BGMZUEKZENQUJY-SSDOTTSWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50133231
Found 14 hits for monomerid = 50133231
Affinity DataKi: 0.100nMAssay Description:In vitro inhibitory constant against [125I]DOI binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Inhibitory constant against cloned human 5-hydroxytryptamine 2A receptor using with [125I]- DOI radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Displacement of [125I]DOI from cloned human 5HT2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H]LSD from human recombinant 5-HT2B receptor expressed in CHO cells by radioligand completion assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [125I]DOI from cloned human 5HT2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory constant against cloned human 5-hydroxytryptamine 2C receptor using with [125I]- DOI radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]LSD from human recombinant 5-HT2A receptor expressed in HEK cells by radioligand completion assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Displacement of [3H]ketanserin from rat prefrontal cortex 5-hydroxytryptamine 2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity to rat cortical membranes at 5-hydroxytryptamine 2 (5-HT2) receptor using [3H]KET as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [125I]DOI from cloned human 5HT2B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory constant against cloned human 5-hydroxytryptamine 2B receptor using with [125I]- DOI radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:Binding affinity for 5-hydroxytryptamine 1 receptor of rat prefrontal cortexMore data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:Evaluated for binding affinity towards rat cortical membranes at 5-hydroxytryptamine 1 receptor binding site by using [3H]-5-HT as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Evaluated for binding affinity towards rat cortical membranes at 5-hydroxytryptamine 1 receptor binding site by using [3H]-5-HT as a radioligand.More data for this Ligand-Target Pair