null
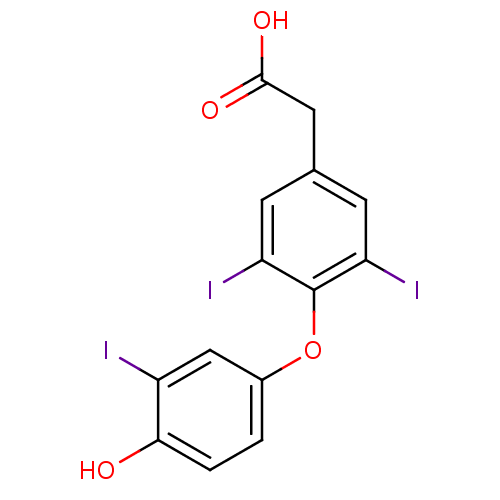
SMILES OC(=O)Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1
InChI Key InChIKey=UOWZUVNAGUAEQC-UHFFFAOYSA-N
PDB links: 13 PDB IDs match this monomer. 2 PDB IDs contain this monomer as substructures. 2 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 18862
Found 9 hits for monomerid = 18862
Affinity DataIC50: 5.00E+3nMAssay Description:In vitro inhibition of bound [125I]L-T3 rat plasma membrane 3,5,3'' L-triiodothyronine receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.48E+4nMAssay Description:Transcriptional activity at human androgen receptor BF3 site stably transfected in eGFP-expressing human LNCAP cells after 5 days by fluorometric ana...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0479nMAssay Description:Inhibition of human thyroid hormone receptor beta 1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0407nMAssay Description:Inhibition of thyroid hormone receptor betaMore data for this Ligand-Target Pair
TargetThyroid hormone receptor alpha(Homo sapiens (Human))
Universidade de S£o Paulo
Curated by ChEMBL
Universidade de S£o Paulo
Curated by ChEMBL
Affinity DataIC50: 0.141nMAssay Description:Inhibition of thyroid hormone receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
TargetThyroid hormone receptor alpha(Homo sapiens (Human))
Universidade de S£o Paulo
Curated by ChEMBL
Universidade de S£o Paulo
Curated by ChEMBL
Affinity DataIC50: 0.140nMpH: 7.0 T: 2°CAssay Description:IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. More data for this Ligand-Target Pair
Affinity DataIC50: 0.0480nMpH: 7.0 T: 2°CAssay Description:IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta.More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)