null
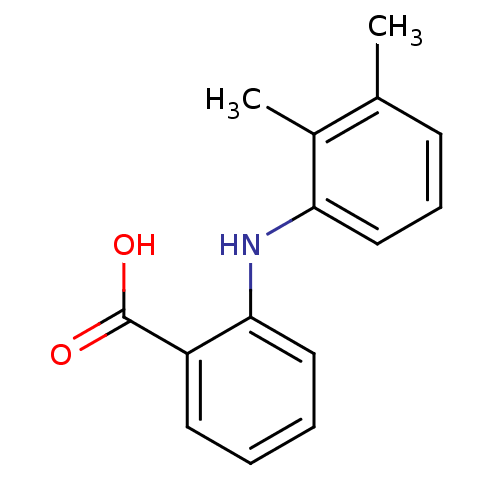
SMILES Cc1cccc(Nc2ccccc2C(O)=O)c1C
InChI Key InChIKey=HYYBABOKPJLUIN-UHFFFAOYSA-N
PDB links: 5 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50134036
Found 11 hits for monomerid = 50134036
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Ljubljana
Curated by ChEMBL
University of Ljubljana
Curated by ChEMBL
Affinity DataKi: 220nMAssay Description:Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Ljubljana
Curated by ChEMBL
University of Ljubljana
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Ljubljana
Curated by ChEMBL
University of Ljubljana
Curated by ChEMBL
Affinity DataKi: 810nMAssay Description:Inhibition of human recombinant AKR1C1 assessed as 1-acenaphthenol oxidation by spectrophotometryMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Ljubljana
Curated by ChEMBL
University of Ljubljana
Curated by ChEMBL
Affinity DataIC50: 560nMAssay Description:Inhibition of AKR1C3 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Ljubljana
Curated by ChEMBL
University of Ljubljana
Curated by ChEMBL
Affinity DataIC50: 6.97E+3nMAssay Description:Inhibition of AKR1C2 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Ljubljana
Curated by ChEMBL
University of Ljubljana
Curated by ChEMBL
Affinity DataIC50: 3.91E+3nMAssay Description:Inhibition of AKR1C1 (unknown origin)More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Affinity DataIC50: 1.03E+5nMAssay Description:Inhibition of COX-2 (unknown origin)More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Dallas Department of Veterans Affairs Medical Center
Curated by PDSP Ki Database
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of COX2 (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)