null
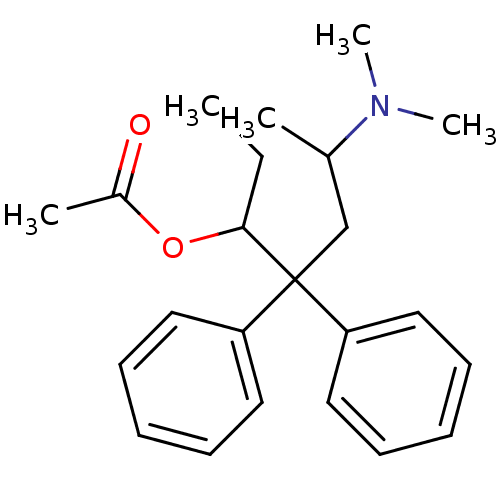
SMILES CCC(OC(C)=O)C(CC(C)N(C)C)(c1ccccc1)c1ccccc1
InChI Key InChIKey=XBMIVRRWGCYBTQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50027391
Found 5 hits for monomerid = 50027391
TargetMu-type opioid receptor(Rattus norvegicus (rat))
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
TargetNorepinephrine transporter(RAT)
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
R.W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Reverse proteomics research institute
Curated by ChEMBL
Reverse proteomics research institute
Curated by ChEMBL
Affinity DataIC50: 2.19E+3nMAssay Description:Inhibitory concentration against potassium channel HERGMore data for this Ligand-Target Pair