null
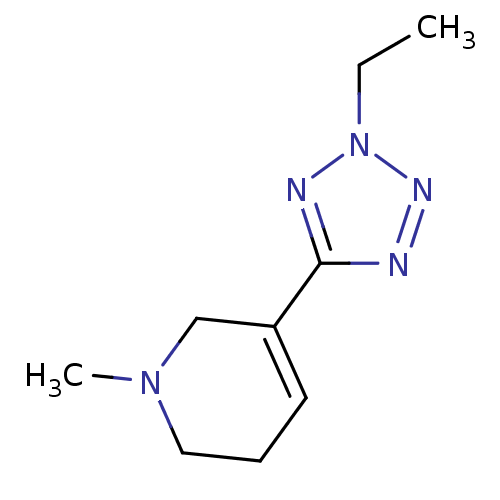
SMILES CCn1nnc(n1)C1=CCCN(C)C1
InChI Key InChIKey=RNMOMKCRCIRYCZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50038210
Found 3 hits for monomerid = 50038210
Affinity DataKi: 36nMAssay Description:In vitro binding affinity for muscarinic M1 receptor by displacing [3H]-Pirenzepine binding on rat brain homogenate.More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 296nMAssay Description:Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assayMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataEC50: >5.10E+4nMAssay Description:M2 agonist activity estimated by depression of isolated guinea pig left atriumMore data for this Ligand-Target Pair