null
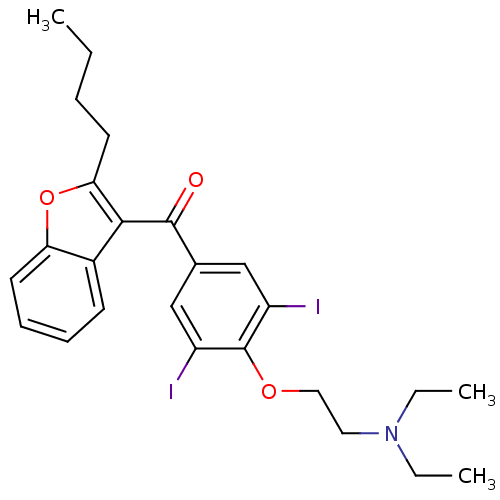
SMILES CCCCc1oc2ccccc2c1C(=O)c1cc(I)c(OCCN(CC)CC)c(I)c1
InChI Key InChIKey=IYIKLHRQXLHMJQ-UHFFFAOYSA-N
PDB links: 8 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 49 hits for monomerid = 18957
Found 49 hits for monomerid = 18957
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
University of Innsbruck
Curated by ChEMBL
University of Innsbruck
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligandMore data for this Ligand-Target Pair
Target3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase(Homo sapiens (Human))
University of Innsbruck
Curated by ChEMBL
University of Innsbruck
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant systemMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1A4(Rattus norvegicus)
Tohoku University School of Medicine
Curated by ChEMBL
Tohoku University School of Medicine
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:TP_TRANSPORTER: inhibition of Digoxin uptake in Xenopus laevis oocytesMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Mus musculus (Mouse))
St. Jude Children's Research Hospital
Curated by ChEMBL
St. Jude Children's Research Hospital
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:TP_TRANSPORTER: increase in Calcein-AM intracellular accumulation in mdr1a-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy of Sciences
Curated by ChEMBL
Bulgarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Concentration giving half of the maximal ATPase activity calculated for the high-affinity binding site of the CHO P-Glycoprotein (P-gp) in two-affini...More data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy of Sciences
Curated by ChEMBL
Bulgarian Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 5.78E+3nMAssay Description:TP_TRANSPORTER: increase in Calcein-AM intracellular accumulation in MDR1-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Mus musculus)
St. Jude Children's Research Hospital
Curated by ChEMBL
St. Jude Children's Research Hospital
Curated by ChEMBL
Affinity DataKi: 1.27E+4nMAssay Description:TP_TRANSPORTER: increase in Calcein-AM intracellular accumulation in mdr1b-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.12E+4nMAssay Description:Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomesMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of voltage-gated L-type Ca channel (species unknown)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of potassium current (Ikr) measured using whole-cell patch clamp experiments in HEK-293 cells stable transfected with hERG cDNAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human Potassium channel HERG expressed in mammalian cellsMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy of Sciences
Curated by ChEMBL
Bulgarian Academy of Sciences
Curated by ChEMBL
Affinity DataEC50: 1.28E+4nMAssay Description:TP_TRANSPORTER: increase in Calcein-AM intracellular accumulation (Calcein-AM: 0.25 uM) in MDR-CEM cellsMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy of Sciences
Curated by ChEMBL
Bulgarian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.56E+4nMAssay Description:TP_TRANSPORTER: inhibition of Digoxin transepithelial transport (basal to apical) (Digoxin: 0.025 uM) in MDR1-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy of Sciences
Curated by ChEMBL
Bulgarian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.25E+4nMAssay Description:TP_TRANSPORTER: inhibition of Daunorubicin transepithelial transport (basal to apical) (Daunorubicin: 0.035 uM) in MDR1-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:Protection against Bacillus anthracis lethal toxin-mediated cytotoxicity in mouse RAW264.7 cells assessed as change in viability after 24 hrs by WST1...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory concentration against potassium channel HERGMore data for this Ligand-Target Pair
TargetCarnitine O-palmitoyltransferase 2, mitochondrial(Homo sapiens (Human))
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CPT2More data for this Ligand-Target Pair
TargetCarnitine O-palmitoyltransferase 1, muscle isoform(Homo sapiens (Human))
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CPT1BMore data for this Ligand-Target Pair
TargetCarnitine O-palmitoyltransferase 1, liver isoform(Homo sapiens (Human))
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibition of human CPT1AMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells by whole cell patch clamp techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 2.06E+5nMAssay Description:Inhibition of Trypanosoma cruzi cruzaine preincubated for 5 mins before substrate addition by fluorescence assay in presence of 0.01% Triton X-100More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of Trypanosoma cruzi cruzaine preincubated for 5 mins before substrate addition by fluorescence assay in absence of Triton X-100More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+4nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.52E+5nMAssay Description:Displacement of C-terminally biotinylated-H3K4me3 (1 to 21 residues) peptide from KDM7B (PHD) (unknown origin) preincubated for 15 mins followed by p...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of KDM5A (L88 to G353 residues) ARID/PHD1/2/3 deletion mutant (unknown origin) demethylation activity preincubated for 10 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+4nMAssay Description:Displacement of C-terminally biotinylated-H3K4me3 (1 to 21 residues) peptide from KDM7C (PHD) (unknown origin) preincubated for 15 mins followed by p...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90E+4nMAssay Description:Inhibition of KDM5A (M1 to L801 residues) PHD2/3 deletion mutant (unknown origin) demethylation activity preincubated for 10 mins followed by peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of KDM5A (M1 to L801 residues) PHD2/3 deletion mutant (unknown origin) demethylation activity preincubated for 10 mins followed by peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+5nMAssay Description:Displacement of C-terminally biotinylated-H3K4me3 (1 to 21 residues) peptide from KDM7A (PHD-JmjC) (unknown origin) preincubated for 15 mins followed...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+5nMAssay Description:Displacement of C-terminally biotinylated-H3K4me3 (1 to 21 residues) peptide from KDM7A (PHD) (unknown origin) preincubated for 15 mins followed by p...More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of Cav1.2 current measured using QPatch automatic path clamp system in CHO cells expressing Cav1.2, beta-2 and alpha-2/delta-1 subunitsMore data for this Ligand-Target Pair
TargetSodium channel protein type 7 subunit alpha(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of Na channel (species unknown)More data for this Ligand-Target Pair
TargetSqualene synthase(Homo sapiens (Human))
Instituto Venezolano de Investigaciones Científicas
Curated by ChEMBL
Instituto Venezolano de Investigaciones Científicas
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibitory activity against Trypanosoma cruzi glycosomal squalene synthaseMore data for this Ligand-Target Pair
TargetSqualene monooxygenase(Homo sapiens (Human))
Instituto Venezolano de Investigaciones Científicas
Curated by ChEMBL
Instituto Venezolano de Investigaciones Científicas
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibitory activity against Trypanosoma cruzi microsomal squalene synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Concentration required to inhibit 50% of binding of [125I]-T3 to human Thyroid hormone receptor beta 1 in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:Concentration required to inhibit 50% of binding of [125I]-T3 to human Thyroid hormone receptor alpha1 in CHO-K1 cellsMore data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetGenome polyprotein(West Nile virus)
University of Pittsburgh Molecular Library Screening Center
Curated by PubChem BioAssay
University of Pittsburgh Molecular Library Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 5.00E+4nMAssay Description:The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMpH: 7.6 T: 2°CAssay Description:IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta.More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMpH: 7.6 T: 2°CAssay Description:IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. More data for this Ligand-Target Pair
Affinity DataEC50: 3.25E+3nMAssay Description:Activation of recombinant human SIRT3 assessed as lysyl deacetylase activity using (Gln-Pro-Lys-Lys(Ac)) peptide substrate by fluorescent assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)