null
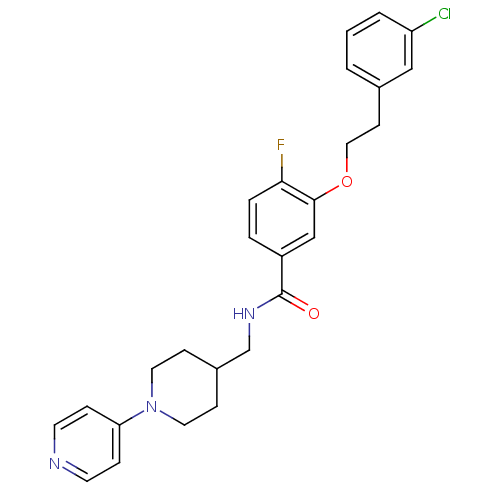
SMILES Fc1ccc(cc1OCCc1cccc(Cl)c1)C(=O)NCC1CCN(CC1)c1ccncc1
InChI Key InChIKey=RHJHCDDSINXMPN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 13634
Found 3 hits for monomerid = 13634
TargetCoagulation factor X(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
Affinity DataKi: 712nMAssay Description:Inhibitory activity against human Coagulation factor XMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universita£t
Curated by ChEMBL
Affinity DataKi: 712nMAssay Description:The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s...More data for this Ligand-Target Pair