null
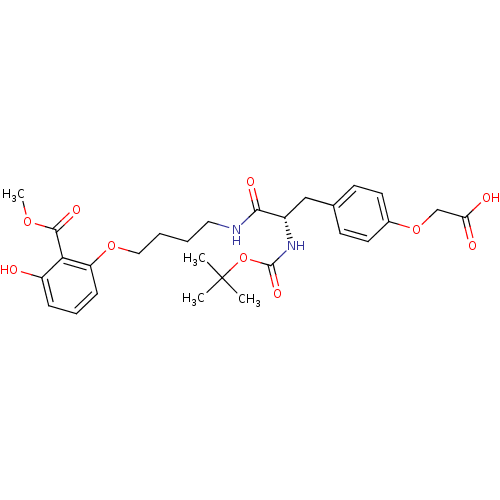
SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)cc1)NC(=O)OC(C)(C)C
InChI Key InChIKey=IKOXRHVKSOJWLA-FQEVSTJZSA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 13986
Found 6 hits for monomerid = 13986
TargetTyrosine-protein phosphatase non-receptor type 22(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Inhibition of T-cell Protein Tyrosine Phosphatase (TCPTP)More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-4.99kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 2.19E+5nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+5nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+5nMAssay Description:Inhibition of Protein-tyrosine phosphatase 1B (PTP1B)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1 [1-298](Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 2.20E+5nM ΔG°: -4.94kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair