null
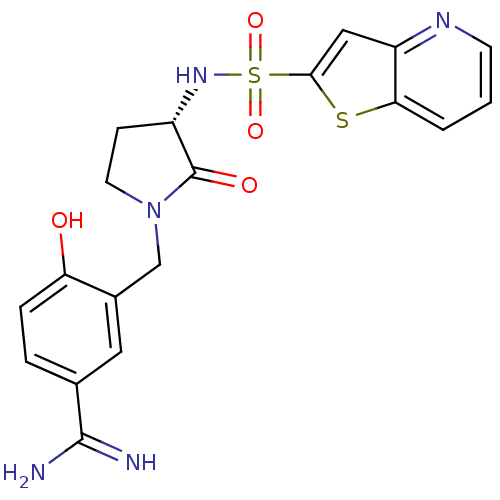
SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1
InChI Key InChIKey=PQJGWYQPOHCEDO-ZDUSSCGKSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 14059
Found 9 hits for monomerid = 14059
Affinity DataKi: 0.700nM ΔG°: -12.4kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Binding affinity against tissue plasminogen activator (t-Pa)More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Binding affinity against trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -8.10kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q25H7DHPPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q25H7DHPPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataKi: 2.30E+3nMAssay Description:Binding affinity against plasminMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nM ΔG°: >-7.28kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.20E+3nMAssay Description:Binding affinity against activated protein C (APC)More data for this Ligand-Target Pair