null
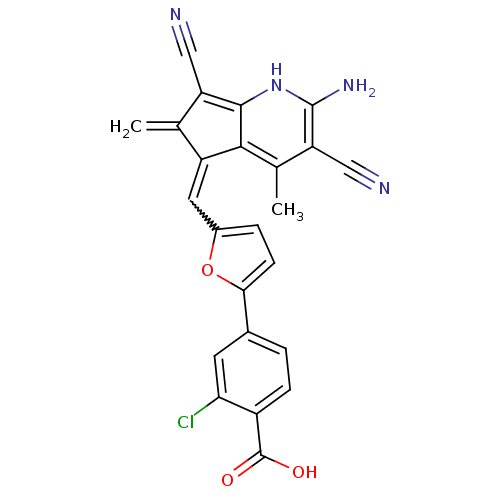
SMILES Cc1c(C#N)c(N)[nH]c2c(C#N)c(=C)c(=Cc3ccc(o3)-c3ccc(C(O)=O)c(Cl)c3)c12
InChI Key InChIKey=YQXKFAZZJMIBIB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 15186
Found 3 hits for monomerid = 15186
Affinity DataKi: 1.10E+3nM ΔG°: -8.12kcal/mole IC50: 2.60E+3nMpH: 7.5 T: 2°CAssay Description:The Z-LYTE assay (Invitrogen Corporation) employs a FRET-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Institute for Medical Research
Curated by ChEMBL
Institute for Medical Research
Curated by ChEMBL
Affinity DataKi: 3.87E+3nMAssay Description:Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studiesMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 4 group A member 1(Homo sapiens (Human))
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 1.65E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair