null
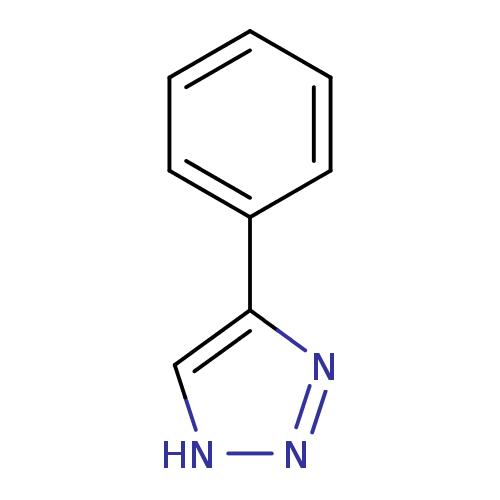
SMILES c1[nH]nnc1-c1ccccc1
InChI Key InChIKey=LUEYUHCBBXWTQT-UHFFFAOYSA-N
PDB links: 2 PDB IDs contain this monomer as substructures. 3 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 17448
Found 13 hits for monomerid = 17448
Affinity DataKi: 70nM ΔG°: -9.66kcal/molepH: 7.5 T: 2°CAssay Description:MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi...More data for this Ligand-Target Pair
Affinity DataKi: 2.25E+4nMAssay Description:Uncompetitive inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate by Dixon...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+5nMAssay Description:Inhibition of mouse recombinant TDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AIMore data for this Ligand-Target Pair
Affinity DataIC50: 8.30E+4nMpH: 6.5Assay Description:Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an...More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+4nMAssay Description:Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+3nMAssay Description:Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysisMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Mus musculus)
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of mouse TDO in P815 clone 12 cells by HPLC analysisMore data for this Ligand-Target Pair
TargetTryptophan 2,3-dioxygenase(Homo sapiens (Human))
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human TDO transfected in mouse P815B clone 19 cells by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+5nMAssay Description:Inhibition of indoleamine-2,3-dioxygenase in human HEK293 cells assessed as N-formylkynurenine level after 5 hrs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.43E+5nMAssay Description:Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Mus musculus)
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
Ludwig Center for Cancer Research of the University of Lausanne
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLCMore data for this Ligand-Target Pair