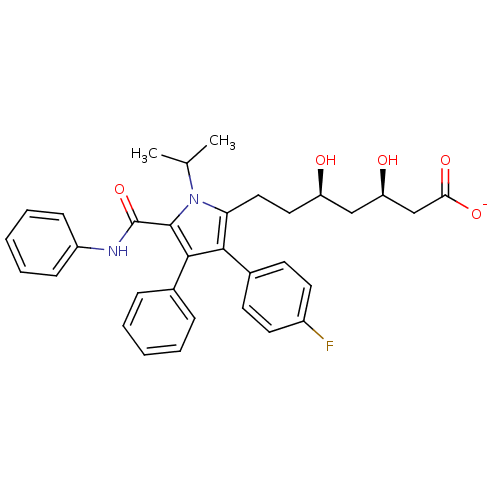
null
SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccccc1)-c1ccccc1)-c1ccc(F)cc1
InChI Key InChIKey=PNRWKVJLHGHICL-KAYWLYCHSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 18374
Found 2 hits for monomerid = 18374
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair