null
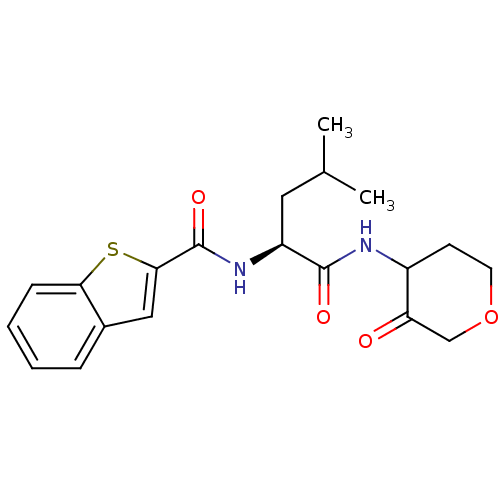
SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)NC1CCOCC1=O
InChI Key InChIKey=SOSDZLZCZHXALY-LOACHALJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 19815
Found 3 hits for monomerid = 19815
Affinity DataKi: 11nM ΔG°: -10.7kcal/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibitory activity of the compound was evaluated against cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair