null
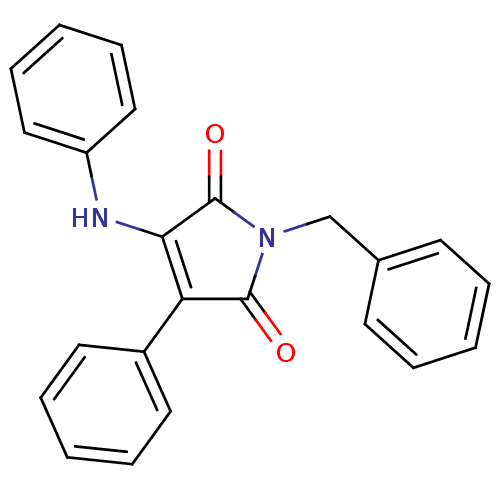
SMILES O=C1N(Cc2ccccc2)C(=O)C(=C1Nc1ccccc1)c1ccccc1
InChI Key InChIKey=GLDDBILAEOMQJI-UHFFFAOYSA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 20157
Found 2 hits for monomerid = 20157
Affinity DataEC50: 70nMpH: 7.5 T: 2°CAssay Description:The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding...More data for this Ligand-Target Pair
Affinity DataEC50: 100nMpH: 7.5 T: 2°CAssay Description:The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding...More data for this Ligand-Target Pair