null
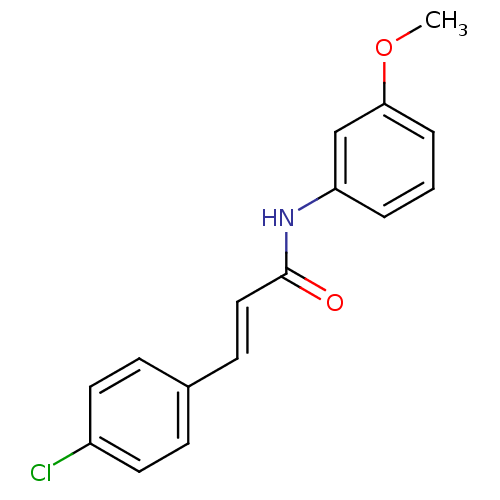
SMILES COc1cccc(NC(=O)\C=C\c2ccc(Cl)cc2)c1
InChI Key InChIKey=RYAMDQKWNKKFHD-JXMROGBWSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 20488
Found 7 hits for monomerid = 20488
Affinity DataEC50: 20nMpH: 7.2 T: 2°CAssay Description:One day before the assay was performed, human TRPV1 expressed in 1321N1 astrocytoma cells were plated onto 96-well assay plates and grown until 2.5 h...More data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Antagonist activity at recombinant human TRPV1 channel expressed in CHO cells assessed as inhibition of capsiacin-induced Ca2+ flux by Fluo-3 dye bas...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Sapienza Universit£ di Roma
Curated by ChEMBL
Sapienza Universit£ di Roma
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of rat brain FAAH assessed as hydrolysis of [14C]AEA to [14C]Ethanolamine incubated for 30 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human TRPM8 expressed in HEK293T cells assessed as blocked of menthol-activated current by whole cell patch clamp electrophysiology rel...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of mouse TRPM1 expressed in HEK293T cells assessed as blocked of capsaicin-activated current at 10 uM by whole cell patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Antagonist activity at recombinant human TRPV1 channel expressed in CHO cells assessed as inhibition of capsiacin-induced Ca2+ flux by Fluo-3 dye bas...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)