null
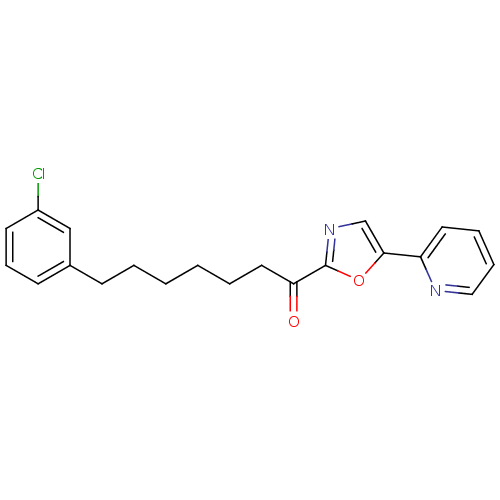
SMILES Clc1cccc(CCCCCCC(=O)c2ncc(o2)-c2ccccn2)c1
InChI Key InChIKey=AJTFTOJFTFVQHK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 23061
Found 3 hits for monomerid = 23061
Affinity DataKi: 0.900nM ΔG°: -12.3kcal/mole IC50: 2nMT: 2°CAssay Description:The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 500nMT: 2°CAssay Description:Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser...More data for this Ligand-Target Pair