null
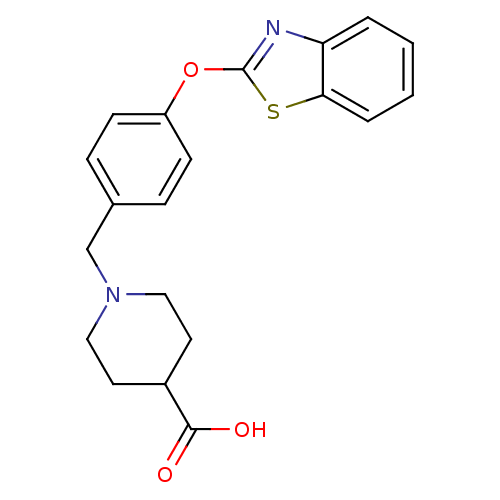
SMILES OC(=O)C1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1
InChI Key InChIKey=BUZBVOKDNAZIIM-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 24239
Found 3 hits for monomerid = 24239
Affinity DataIC50: 11nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Research& Development LLC
Curated by ChEMBL
Janssen Research& Development LLC
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of dofetilide binding to human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Janssen Research& Development LLC
Curated by ChEMBL
Janssen Research& Development LLC
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)