null
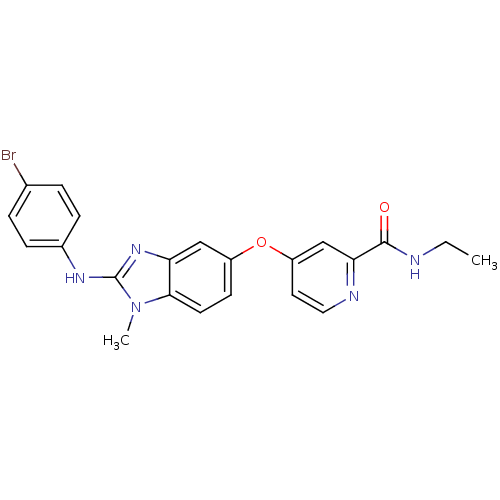
SMILES CCNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1
InChI Key InChIKey=UVIZFSBFHNHFCO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 26035
Found 2 hits for monomerid = 26035
Affinity DataIC50: 13nMAssay Description:To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated...More data for this Ligand-Target Pair