null
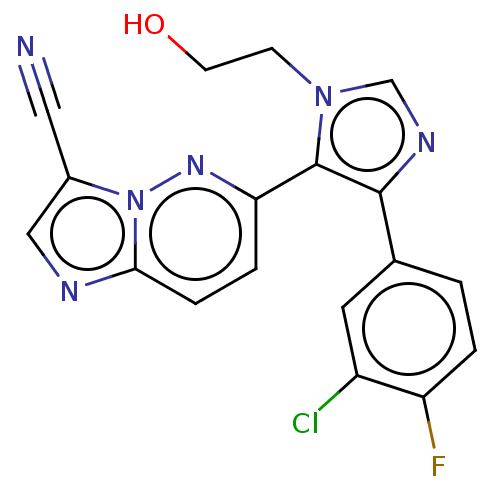
SMILES OCCn1cnc(c1-c1ccc2ncc(C#N)n2n1)-c1ccc(F)c(Cl)c1
InChI Key InChIKey=VZZBCNXVZFAIQX-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 282825
Found 21 hits for monomerid = 282825
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of TGFBR1 in human whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylationMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Mus musculus)
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of TGFBR1 in mouse whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylationMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-2(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of wild type His-tagged TGFBR2 kinase domain (unknown origin) incubated for 1 hr by HTRF analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of TGFbetaR1 in TGFbeta-stimulated mink Mv1Lu cells assessed as reduction in SMAD nuclear translocation preincubated for 1 hr followed by ...More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of TGFBR1 in TGFbeta-stimulated human NHLF cells assessed as reduction in SMAD2 nuclear translocationMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of TGFbetaR1 in TGFbeta-stimulated human T cells assessed as reduction in SMAD3 phosphorylation preincubated for 1 hr followed by TGFbeta-...More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Mus musculus)
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of TGFbetaR1 in TGFbeta-stimulated mouse NIH3T3 cells assessed as reduction in SMAD3 phosphorylationMore data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataEC50: >2.40E+4nMAssay Description:Activation of PXR (unknown origin)More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of PDE4 (unknown origin) by HTRF assayMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of His-tagged TGFBR1 kinase domain T204D mutant (unknown origin) incubated for 1 hr by HTRF analysisMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of TGFbetaR1 in anti-CD3/anti-CD28/IL-2/TGFbeta -stimulated human Treg cells assessed as downregulation of FOXP3 expression incubated for ...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERG by electrophysiology analysisMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or...More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMpH: 7.4 T: 2°CAssay Description:Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)