null
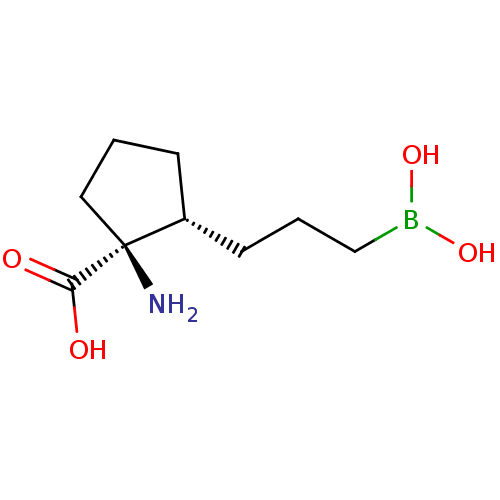
SMILES N[C@]1(CCC[C@@H]1CCCB(O)O)C(O)=O
InChI Key InChIKey=HBEGNHDSKKYWSU-APPZFPTMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 290365
Found 8 hits for monomerid = 290365
Affinity DataIC50: 625nMpH: 7.4 T: 2°CAssay Description:Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp...More data for this Ligand-Target Pair
Affinity DataIC50: 625nMpH: 7.4 T: 2°CAssay Description:Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp...More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assayMore data for this Ligand-Target Pair
Affinity DataIC50: 625nMAssay Description:Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp...More data for this Ligand-Target Pair