null
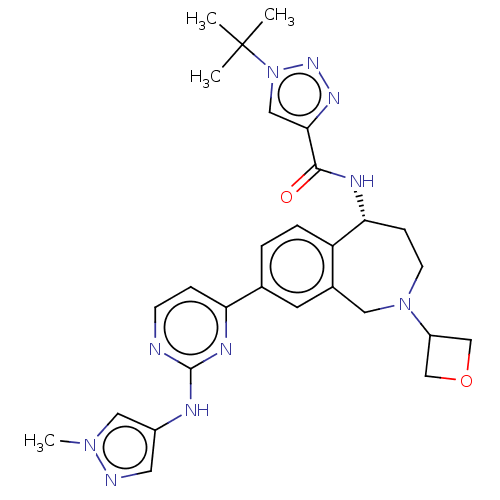
SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1
InChI Key InChIKey=JSAQBOQCZJHWMA-XMMPIXPASA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 26 hits for monomerid = 324284
Found 26 hits for monomerid = 324284
Affinity DataIC50: 1nMAssay Description:The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1nMAssay Description:The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of BTK phosphorylation in human whole blood assessed as reduction in BTK phosphorylation incubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of CD69 in human whole blood preincubated for 30 mins followed by anti-human IgD stimulation and measured after 18 to 22 hrs by flow cytom...More data for this Ligand-Target Pair
Affinity DataKd: 0.0700nMAssay Description:Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 660nMAssay Description:Binding affinity to wild-type human partial length AURKA (E122 to K401 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 88nMAssay Description:Binding affinity to wild-type human full length BMX (M1 to H675 residues) expressed in bacterial expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 240nMAssay Description:Binding affinity to wild-type human partial length CDKL2 (M1 to D327 residues) expressed in bacterial expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: >3.00E+3nMAssay Description:Binding affinity to wild-type human full length LZK (M1 to W966 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 200nMAssay Description:Binding affinity to wild-type human full length MEK2 (M1 to V400 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 420nMAssay Description:Binding affinity to wild-type human partial length PIP5K1C (M1 to T668 residues) expressed in mammalian expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 320nMAssay Description:Binding affinity to wild-type human partial length SRC (L240 to L536 residues) expressed in bacterial expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 240nMAssay Description:Binding affinity to wild-type human partial length TEC (L341 to D620 residues) expressed in bacterial expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataKd: 40nMAssay Description:Binding affinity to wild-type human partial length TIE1 (T819 to A1138 residues) expressed in bacterial expression system by Kinomescan methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human ERG by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human Cav1.2More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of tonic human Nav1.5More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of phasic human Nav1.5More data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of BTK in human Ramos cells assessed as reduction in PLCgamma2 phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Inhibition of BTK in anti-IgM stimulated human PBMC cells assessed as reduction in PLCgamma2 phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:Inhibition of CD69 (unknown origin) assessed as reduction in BCR mediated B cell activationMore data for this Ligand-Target Pair
Affinity DataIC50: 82nMAssay Description:Inhibition of CD63 (unknown origin) assessed as FcepsilonR induced basophil activationMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human BTK assessed as reduction in OVA323-329 specific T cells proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 505nMAssay Description:The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)