null
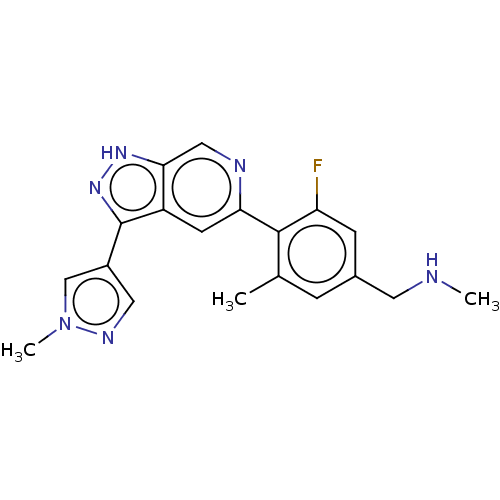
SMILES CNCc1cc(C)c(c(F)c1)-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1
InChI Key InChIKey=YZXSIGGVKBZQGJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 414751
Found 9 hits for monomerid = 414751
Affinity DataKi: 2.70nMAssay Description:In vitro inhibitory concentration against rat liver dihydrofolate reductaseMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: <100nMAssay Description:A stock solution of 1 mM test compound was prepared in DMSO. The compound plate was prepared by 3-fold and 11-point serial dilutions. 0.1 μL of ...More data for this Ligand-Target Pair
Affinity DataKi: <100nMAssay Description:A stock solution of 1 mM test compound was prepared in DMSO. The compound plate was prepared by 3-fold and 11-point serial dilutions. 0.1 μL of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:In vitro inhibitory concentration against rat liver dihydrofolate reductaseMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 91nMAssay Description:In vitro inhibitory concentration against rat liver dihydrofolate reductaseMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2.50E+4nMAssay Description:In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.51E+3nMAssay Description:Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptorMore data for this Ligand-Target Pair
Target InfoPDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 7.47E+3nMAssay Description:Antagonist potency against muscarinic receptors was assed by antagonism of carbachol induced inhibition of electrically stimulated guinea pig atriaMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.62E+3nMAssay Description:Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptorMore data for this Ligand-Target Pair
In DepthDetails