null
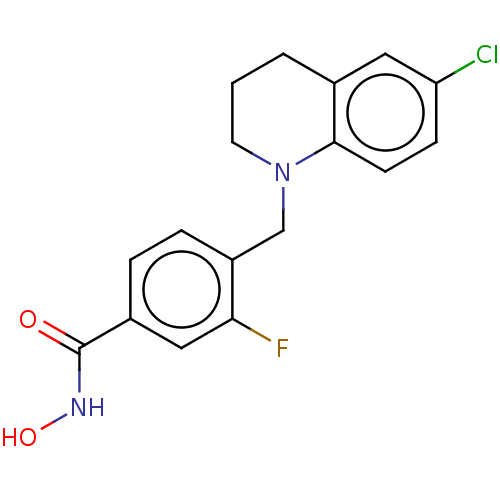
SMILES ONC(=O)c1ccc(CN2CCCc3cc(Cl)ccc23)c(F)c1
InChI Key InChIKey=YWGJBRYPUNDFFF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 417065
Found 4 hits for monomerid = 417065
TargetHistone deacetylase 1(Homo sapiens (Human))
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS
US Patent
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS
US Patent
Affinity DataIC50: 2.12E+4nMAssay Description:The effectiveness, or potency, of a present HDACI with respect to inhibiting the activity of an HDAC is measured by an IC50 value. The quantitative I...More data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+4nMAssay Description:Inhibition of full length recombinant human HDAC6 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr...More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS
US Patent
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS
US Patent
Affinity DataIC50: 12nMAssay Description:Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr...More data for this Ligand-Target Pair
Affinity DataIC50: 12.4nMAssay Description:The effectiveness, or potency, of a present HDACI with respect to inhibiting the activity of an HDAC is measured by an IC50 value. The quantitative I...More data for this Ligand-Target Pair