null
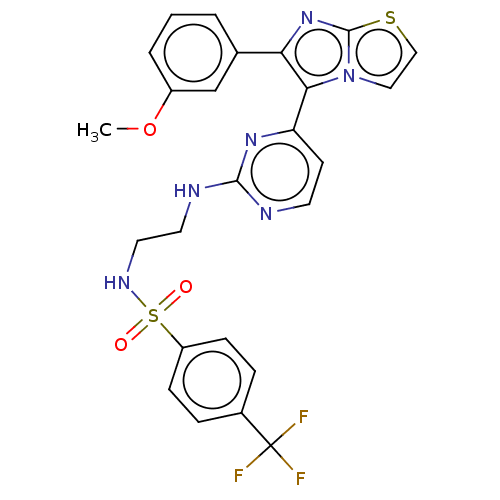
SMILES COc1cccc(c1)-c1nc2sccn2c1-c1ccnc(NCCNS(=O)(=O)c2ccc(cc2)C(F)(F)F)n1
InChI Key InChIKey=ZDXCHNZCVKMKQZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 435720
Found 17 hits for monomerid = 435720
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 687nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 9.25E+3nMAssay Description:Inhibition of NTRK2 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 687nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 687nMAssay Description:Inhibition of human wild type BRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 98nMAssay Description:Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co...More data for this Ligand-Target Pair
Affinity DataIC50: 142nMAssay Description:Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count...More data for this Ligand-Target Pair
Affinity DataIC50: 9.85E+3nMAssay Description:Inhibition of ABL1 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of ERN1 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Inhibition of FGFR1 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of FLT3 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.58E+3nMAssay Description:Inhibition of HIPK1 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of MAPK8 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of MAPK11 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inhibition of MAPK14 (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.11E+3nMAssay Description:Inhibition of ZAK (unknown origin) incubated for 1 hr by qPCR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair