null
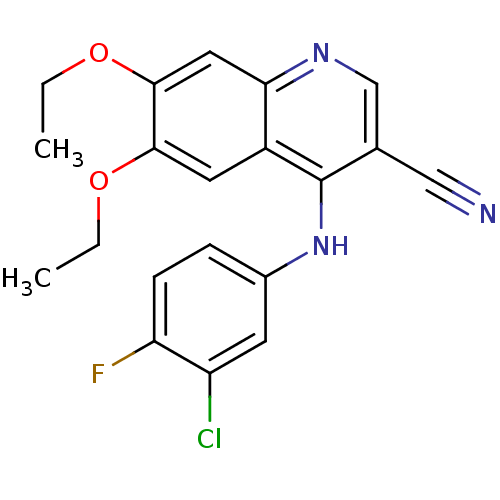
SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(F)c(Cl)c3)c2cc1OCC
InChI Key InChIKey=VJTVBWNBERHDKM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 4444
Found 2 hits for monomerid = 4444
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 977nMAssay Description:Inhibition autophosphorylation of EGFR in human DiFi cells after 2 hrs by ELISAMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 980nMpH: 7.4 T: 2°CAssay Description:The EGF-R kinase autophosphorylation activity was measured by DELFIA/time-resolved fluorometry with excitation at 340 nm and emission at 615 nm. Po...More data for this Ligand-Target Pair