null
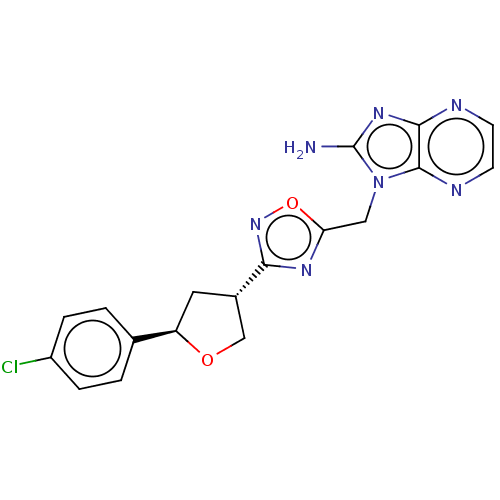
SMILES Nc1nc2nccnc2n1Cc1nc(no1)[C@@H]1CO[C@H](C1)c1ccc(Cl)cc1
InChI Key InChIKey=OOABNTZYAYDDFY-WCQYABFASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 452634
Found 2 hits for monomerid = 452634
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech, Inc.
US Patent
Genentech, Inc.
US Patent
Affinity DataIC50: 3.80nMAssay Description:The IC50 (effective concentration) of compounds on the human TRPA1 channel was determined using a FLIPR Tetra instrument. CHO cells expressing TRPA1 ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Genentech, Inc.
US Patent
Genentech, Inc.
US Patent
Affinity DataIC50: 10.8nMAssay Description:The IC50 (effective concentration) of compounds on the human TRPA1 channel was determined using a FLIPR Tetra instrument. CHO cells expressing TRPA1 ...More data for this Ligand-Target Pair