null
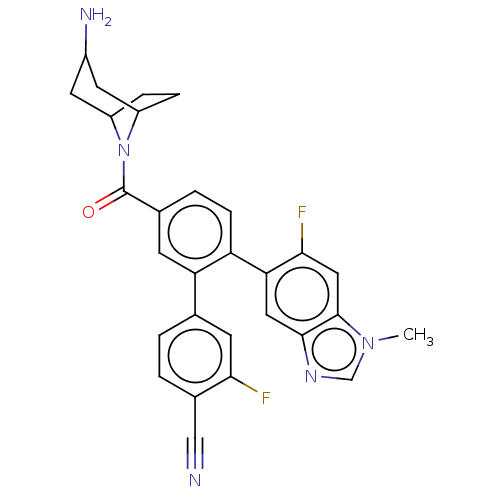
SMILES Cn1cnc2cc(c(F)cc12)-c1ccc(cc1-c1ccc(C#N)c(F)c1)C(=O)N1C2CCC1CC(N)C2
InChI Key InChIKey=BVTCVRWRLJAXGA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 456387
Found 3 hits for monomerid = 456387
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Taiho Pharmaceutical Co., Ltd.
US Patent
Taiho Pharmaceutical Co., Ltd.
US Patent
Affinity DataIC50: 0.210nMAssay Description:The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Taiho Pharmaceutical Co., Ltd.
US Patent
Taiho Pharmaceutical Co., Ltd.
US Patent
Affinity DataIC50: 0.290nMAssay Description:The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Taiho Pharmaceutical Co., Ltd.
US Patent
Taiho Pharmaceutical Co., Ltd.
US Patent
Affinity DataIC50: 0.290nMAssay Description:The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w...More data for this Ligand-Target Pair