null
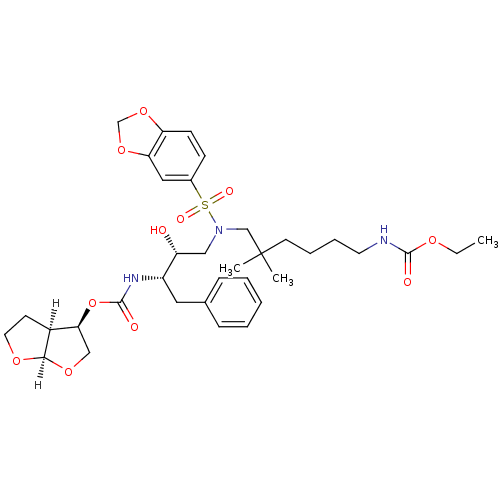
SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)(C)CCCCNC(=O)OCC)S(=O)(=O)c1ccc2OCOc2c1
InChI Key InChIKey=UZCJMLRGHFWFPA-OLNQLETPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 4688
Found 4 hits for monomerid = 4688
Affinity DataKi: 0.000165nM ΔG°: -17.7kcal/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
GlaxoSmithKline
GlaxoSmithKline
Affinity DataKi: 0.00120nM ΔG°: -16.5kcal/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
GlaxoSmithKline
GlaxoSmithKline
Affinity DataKi: 0.00430nM ΔG°: -15.8kcal/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
GlaxoSmithKline
GlaxoSmithKline
Affinity DataKi: 0.00460nM ΔG°: -15.7kcal/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair