null
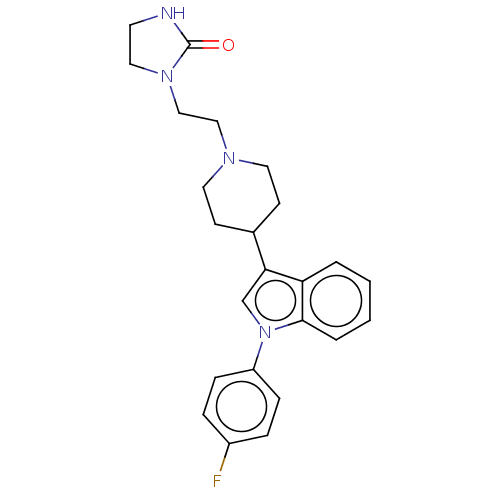
SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2ccccc12
InChI Key InChIKey=QPOLRENOYJQNEP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50001778
Found 8 hits for monomerid = 50001778
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TCG Lifesciences Ltd.
Curated by ChEMBL
TCG Lifesciences Ltd.
Curated by ChEMBL
Affinity DataIC50: 6.92nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Displacement of [3H]-spiperone from Dopamine receptor D2 from rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Tested for the inhibition of [3H]-spiperone binding to dopamine D2 receptorMore data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rattus norvegicus (rat))
H. Lundbeck A/S
Curated by ChEMBL
H. Lundbeck A/S
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]prazosin from Alpha-1 adrenergic receptor of whole rat brain membranesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TCG Lifesciences Ltd.
Curated by ChEMBL
TCG Lifesciences Ltd.
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of K+ channel activity in CHO cells expressing HERG Kv11.1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0820nMAssay Description:Inhibition of [3H]ketanserin binding to rat 5-hydroxytryptamine 2 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMAssay Description:Displacement of [3H]ketanserin from 5-hydroxytryptamine 2 receptor from rat cortical membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Binding affinity at dopamine D2 receptor by [3H]-Spiperone displacement.More data for this Ligand-Target Pair