null
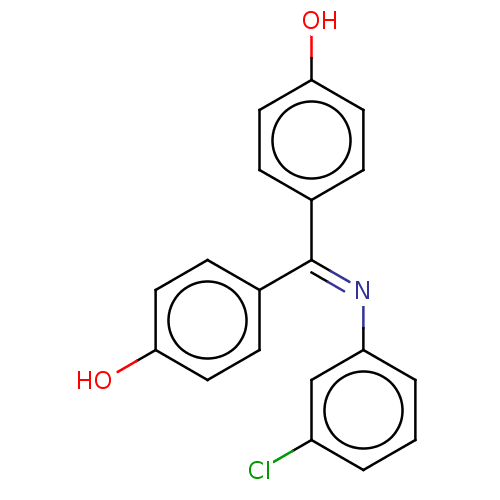
SMILES [#8]-c1ccc(cc1)-[#6](=[#7]/c1cccc(Cl)c1)\c1ccc(-[#8])cc1
InChI Key InChIKey=JZYCMXQTCPCMIE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50005592
Found 2 hits for monomerid = 50005592
TargetEstrogen receptor(Homo sapiens (Human))
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Agonist activity at ERalpha (unknown origin) expressed in human HepG2 cells assessed as transcriptional activation after 24 hrs by ERE-luciferase rep...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair