null
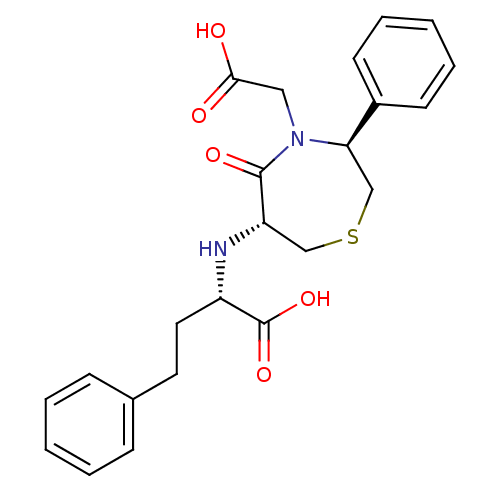
SMILES OC(=O)CN1[C@H](CSC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O)c1ccccc1
InChI Key InChIKey=HGENZIJEOCHEPV-SLFFLAALSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50023301
Found 2 hits for monomerid = 50023301
Affinity DataIC50: 78nMAssay Description:Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrateMore data for this Ligand-Target Pair