null
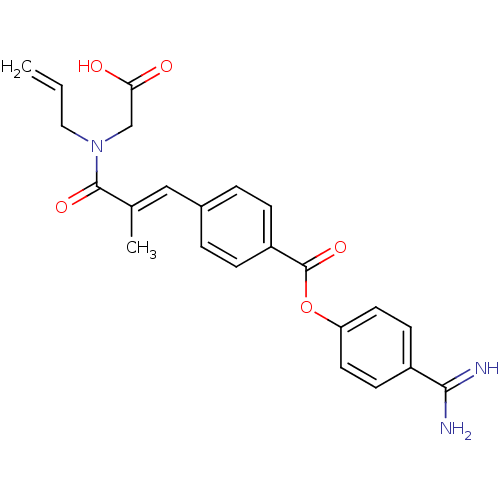
SMILES C\C(=C/c1ccc(cc1)C(=O)Oc1ccc(cc1)C(N)=N)C(=O)N(CC=C)CC(O)=O
InChI Key InChIKey=YZNRBKWDIOXEAU-FYWRMAATSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50031707
Found 8 hits for monomerid = 50031707
Affinity DataIC50: 97nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Pro-Phe-Arg-pNA) for plasma kallikrein in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Val-Leu-Arg-pNA) for pancreatic kallikrein in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (MeO-Suc-Ala-Ala-Pro-Met-pNA) for cathepsin G in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 4.13nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (Boc-Phe-Ser-Arg-AMC) for trypsin in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Val-Leu-Lys-pNA) for plasmin in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (MeO-Suc-Ala-Ala-Pro-Val-pNA) for sputum elastase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (Suc-Ala-Ala-Pro-Phe-pNA) for chymotrypsin in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+4nMAssay Description:Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Phe-Pip-Arg-pNA) thrombin in vitro.More data for this Ligand-Target Pair