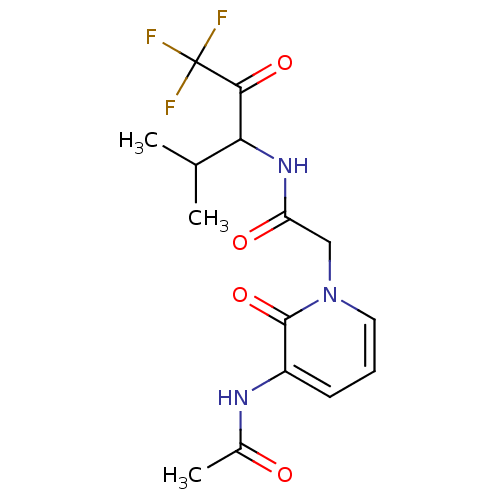
null
SMILES CC(C)C(NC(=O)Cn1cccc(NC(C)=O)c1=O)C(=O)C(F)(F)F
InChI Key InChIKey=QIOIVBRPECJGDP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50037115
Found 3 hits for monomerid = 50037115
Affinity DataKi: 2.80E+3nMAssay Description:Inhibition of Human Leukocyte Elastase (HLE)More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:The compound was tested in vitro for ability to inhibit human leukocyte elastase activityMore data for this Ligand-Target Pair