null
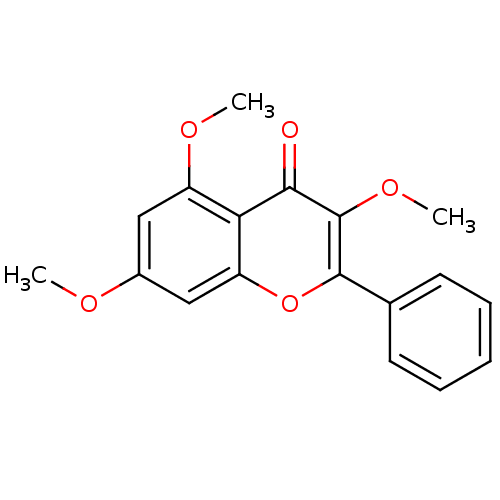
SMILES COc1cc(OC)c2c(c1)oc(-c1ccccc1)c(OC)c2=O
InChI Key InChIKey=CBTHKWVPSIGKMI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50049384
Found 11 hits for monomerid = 50049384
Affinity DataKi: 509nMAssay Description:Displacement of specific [3H]-PIA binding from adenosine A1 receptor in rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 509nMAssay Description:Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H]-PIA displacement.More data for this Ligand-Target Pair
Affinity DataKi: 513nMAssay Description:Ability to displace [3H]N6-phenylisopropyladenosine binding from adenosine A1 receptor.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 513nMAssay Description:Ability to displace [3H]N6-phenylisopropyladenosine binding from adenosine A1 receptor.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 831nMAssay Description:Ability to displace [125I]-AB-MECA binding from adenosine A3 receptor.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 831nMAssay Description:Ability to displace [125I]-AB-MECA binding from adenosine A3 receptor.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 1.21E+3nMAssay Description:Binding affinity against human adenosine A3 receptor in HEK293 cells using [125I]-AB-MECA 21680 radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.21E+3nMAssay Description:Displacement of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in HEK-293 cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 6.45E+3nMAssay Description:Affinity at Adenosine A2A receptor in rat striatal membranes by [3H]- CGS 21680 displacement.More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 6.46E+3nMAssay Description:Ability to displace [3H]-CGS- 21680 binding from adenosine A2A receptor.Checked by AuthorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 6.46E+3nMAssay Description:Ability to displace [3H]-CGS- 21680 binding from adenosine A2A receptor.Checked by AuthorMore data for this Ligand-Target Pair