null
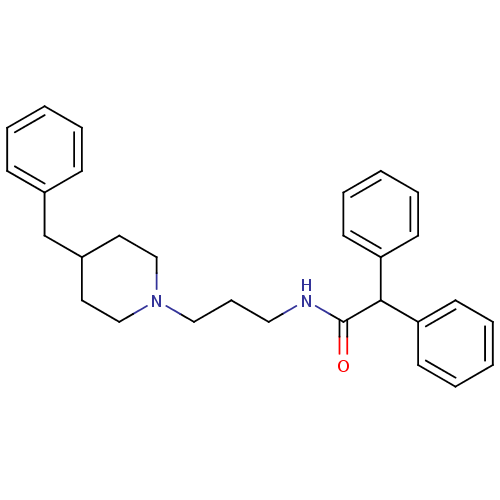
SMILES O=C(NCCCN1CCC(Cc2ccccc2)CC1)C(c1ccccc1)c1ccccc1
InChI Key InChIKey=DKXNWXXUHISEHY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50051985
Found 7 hits for monomerid = 50051985
TargetSodium channel protein type 2 subunit alpha(Rattus norvegicus)
Warner-Lambert Company
Curated by ChEMBL
Warner-Lambert Company
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibitory activity against type IIA sodium channel in CNaIIA-1 cell line expressed in CHO cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Chonnam National University
Curated by ChEMBL
Chonnam National University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of [3H]-5-hydroxytryptamine reuptake in human SERT expressed in HEK293 cells by microbeta liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Chonnam National University
Curated by ChEMBL
Chonnam National University
Curated by ChEMBL
Affinity DataIC50: 2.21E+3nMAssay Description:Inhibition of [3H]-5-norepinephrine reuptake in human NET expressed in HEK293 cells by microbeta liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Chonnam National University
Curated by ChEMBL
Chonnam National University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of [3H]serotonin uptake at SERT (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Chonnam National University
Curated by ChEMBL
Chonnam National University
Curated by ChEMBL
Affinity DataIC50: 1.07E+3nMAssay Description:Inhibition of [3H]dopamine uptake at DAT (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Chonnam National University
Curated by ChEMBL
Chonnam National University
Curated by ChEMBL
Affinity DataIC50: 2.21E+3nMAssay Description:Inhibition of [3H]norepinephrine uptake at NET (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Chonnam National University
Curated by ChEMBL
Chonnam National University
Curated by ChEMBL
Affinity DataIC50: 1.07E+3nMAssay Description:Inhibition of yeast Farnesyl transferaseMore data for this Ligand-Target Pair