null
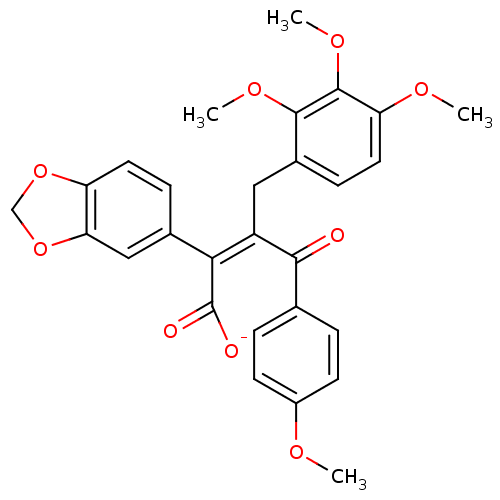
SMILES COc1ccc(cc1)C(=O)C(\Cc1ccc(OC)c(OC)c1OC)=C(/C([O-])=O)c1ccc2OCOc2c1
InChI Key InChIKey=KVMLOIHJNDCBKI-GFMRDNFCSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50057153
Found 2 hits for monomerid = 50057153
Affinity DataIC50: 1.10nMAssay Description:Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of Endothelin B receptor mediated (ET-3) release of arachidonic acid from human cloned receptors expressed in CHO-K1 cellsMore data for this Ligand-Target Pair