null
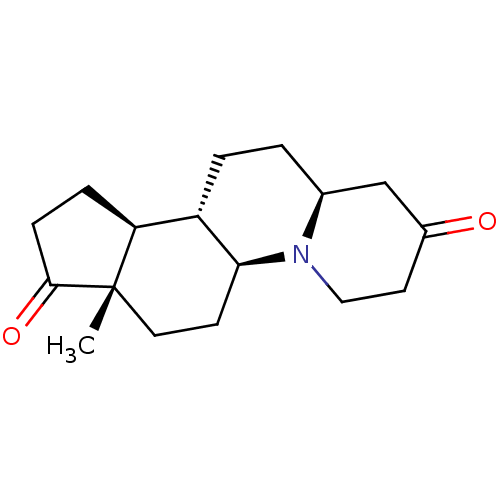
SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4CC(=O)CCN34)[C@@H]1CCC2=O
InChI Key InChIKey=VWYXLVRVZRLBLR-YLVYSCNESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50057289
Found 3 hits for monomerid = 50057289
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
Universit£ di Firenze
Curated by ChEMBL
Universit£ di Firenze
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against 5-alpha Reductase-2 on human prostate homogenates from surgically derived benign hyperplastic tissueMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
Universit£ di Firenze
Curated by ChEMBL
Universit£ di Firenze
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition against 5 alpha R-2 in human prostate homogenate relative to finasterideMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
Universit£ di Firenze
Curated by ChEMBL
Universit£ di Firenze
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against human 5-alpha Reductase-1 expressed in DU-145 cellsMore data for this Ligand-Target Pair